Inositol phosphates analogs as therapeutics
Clostridioides difficile
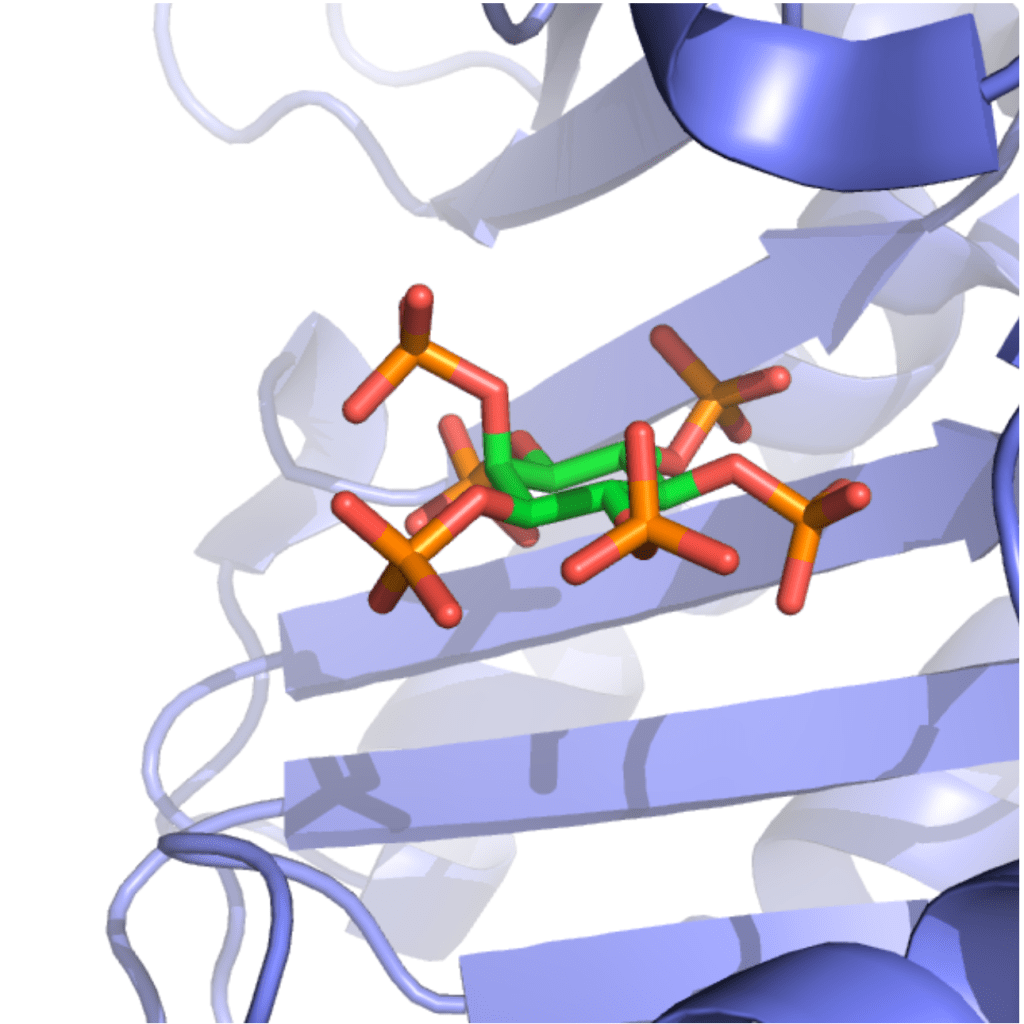
Clostridioides difficile is a bacterium which causes severe and potentially fatal intestinal infections. The US Centers for Disease Control and Prevention has called C. difficile one of the five most urgent resistant pathogens. C. difficile infection (CDI) is caused by the use of antibiotics because antibiotics deplete the gut microbiota that normally protects against CDI. As a result, current antibiotic treatments plague patients with disease recurrence. Therefore, novel therapies are urgently needed to deal with CDI (Ivarsson et al. Drug Discov. Today 2015). The pathogenesis of C. difficile is driven by two large protein toxins: TcdA and TcdB, the latter being the primary virulence determinant in human infections. Targeting TcdB is an attractive therapeutic approach since it avoids the use of antibiotics. We have recently published an innovative approach using small molecules to inactivate TcdB (Ivarsson et al. Cell Chem. Biol. 2019; Cummer et al. J. Med. Chem. 2024). We hypothesized that analogues of inositol hexakisphosphate (IP6), a natural co-factor responsible for TcdB auto-processing inside human intestinal cells, would be able to pre-emptively induce auto-processing in the gut lumen, prior to toxin cell uptake. We synthesized inositol phosphate analogues that effectively induced toxin cleavage in the presence of intestinal concentrations of calcium. Gratifyingly, our approach reduced mortality in a fulminant mouse CDI model when administered orally. This was the first demonstration that auto-processing of a toxin can be exploited for therapeutic purposes. In collaboration with computational chemist Dr. Bettina Keller, we studied the allosteric transition of the toxin’s protease domain when it binds IP6 and identified important residues (Finn et al. PNAS 2025). The thiophosphates analog of IP6 is an interesting bioisostere that binds other IP6-binding bacterial effector such as AvrA from Salmonella enterica serovar Typhimurium and VopA from Vibrio parahaemolyticus. (Cummer et al. RSC Chem. Biol. 2025).
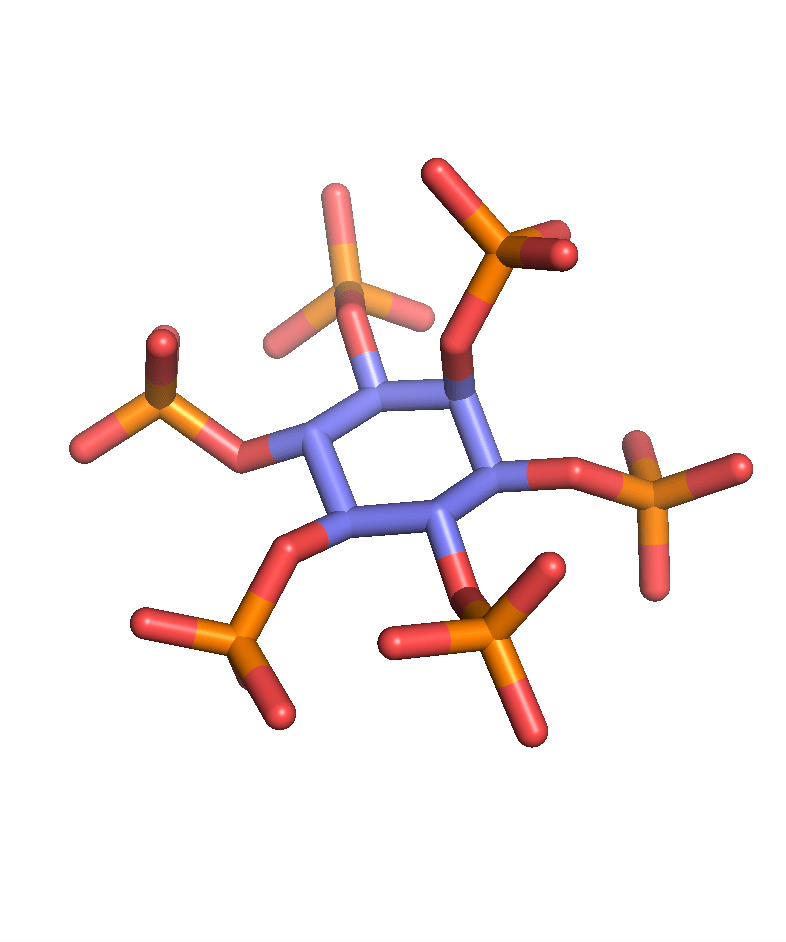
Calcium binding
We have also exploited the inorganic calcium phosphate binding property of inositol phosphates to bind nanoparticles for drug delivery (Huang et al. ACS Appl. Mater. Interfaces 2017). Together with colleagues at Inositec, we have developed INS-3001, a pathological calcification inhibitor that was tested in phase I clinical trial (Schantl et al. Nat. Commun. 2020).
Targeting the Gut Microbiota
The human gut microbiota is essential to our physiology and has recently been linked to many conditions, including obesity, inflammatory bowel diseases, and even neurodegenerative diseases. Recently, the gut microbiota has been convincingly implicated in the efficacy of immune-checkpoint inhibitors (ICIs) in cancer. Immunotherapy that targets immune checkpoints like programmed cell death 1 (PD-1) has revolutionized the way several cancers are treated, but a large proportion of patients do not respond to the treatment. Our colleagues have shown that non-responders had an altered microbiota, which could transfer the phenotype of treatment resistance when transplanted into germ-free mice. Importantly, the administration of immuno-stimulatory bacteria to these mice re-sensitized them to ICIs, suggesting that gut microbiota manipulations could increase cancer immunotherapy response (Elkrief et al. Nat. Rev. Drug Discov. 2025).
Polyphenols
Together with the teams of Dr. Routy and Dr. Marette, we demonstrated that castalagin, an ellagitannin found in the camu-camu berry has an impact on the human gut microbiota in a way that promotes response to immunotherapy (Messaoudène et al. Cancer Discovery 2022). We are currently developing this therapeutic strategy further. A phase I clinical trial is currently underway by our collaborator to evaluate camu-camu in the context of cancer immunotherapy (NCT05303493), as well as investigators at the City of Hope Medical Center in California (NCT06049576).
We are notably interested in understanding the bi-directional interactions between dietary ellagitannins, such as castalagin, and the gut microbiota. Notably, the genes involved in the metabolism of ellagitannins by the human gut microbiome are still unknown. We are applying multi-omics methods to discover the bacteria and genes responsible so we can understand better the link between dietary polyphenols and health (Pidgeon et al. Nat. Commun. 2025; Pidgeon et al. npj Biofilms and Microbiomes 2025).
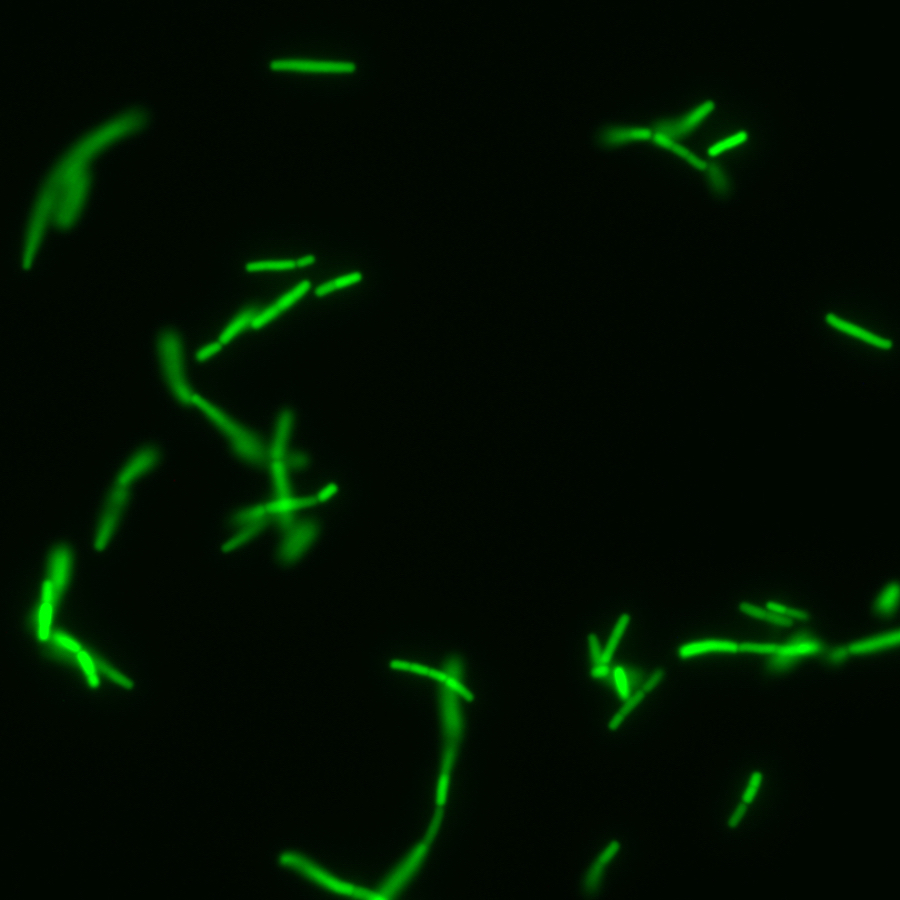
Glycan prebiotics
Complex glycans and polysaccharides contained in our diet go through the digestive tract un-digested by the host and are a major energy source for our gut microbiota. As such, microbiota-accessible glycans are a major driver of the microbiome composition and diversity. It is therefore possible to use glycans to manipulate the composition of the gut microbiota (Altamura et al. Curr. Op. Chem. Biol. 2020). We aim to discover glycan prebiotics that will promote anti-tumor immunity and improve clinical response in cancer patients. To that end, we have developed chemical biology tools to better understand gut bacteria metabolism of glycans together with our collaborator Dr. Corinne Maurice. We used fluorescent glycans to metabolically label consuming bacteria, which were then sorted by fluorescence-activated cell sorting (FACS) and sequenced or isolated (Dridi et al. Nat. Commun. 2023). This pipeline was useful to explore the impact of acarbose, a drug used in diabetes, on glycan metabolism by gut bacteria (Lui et al. ACS Chem. Biol. 2023). In addition, we developed a metatranscriptomics method that could highlight genes and bacterial taxa responding to the presence of a glycan in a microbiome sample. The method allowed us to identify new glycan utilizers that had not been reported before (Prattico et al. mSphere 2025).